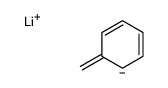
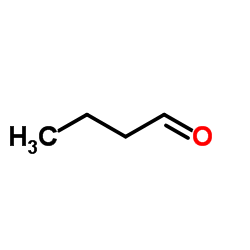
|
~71% |
|
~87% |
|
~% |
|
~% |
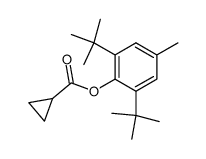
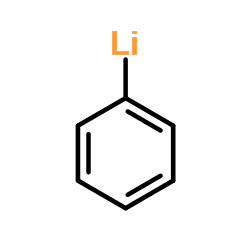

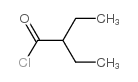
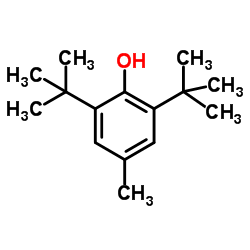
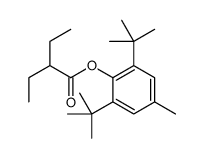
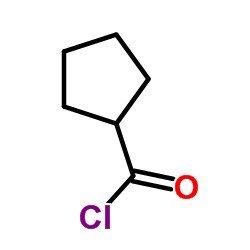
![1-[1-(1-hydroxybutyl)cyclopentyl]-2-phenylethanone Structure](https://image.chemsrc.com/caspic/151/97234-38-3.png)