|
~% |
|
~% |
|
~% |
|
~% |
|
~87% |
|
~79% |



![(2S,3R,4R)-2-acetamido-3-acetoxy-2-acetoxymethyl-4-[(E)-10'-oxohexadec-2'-en-1'-yl]-4-butanolide Structure](https://image.chemsrc.com/caspic/444/37818-01-2.png)
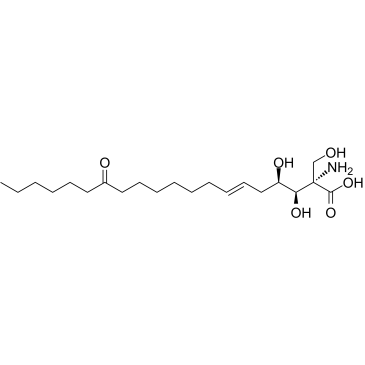
![(1S,5R,6R)-1-hydroxymethyl-6-[(E)-10-oxohexadec-2-en-1-yl]-2-aza-4,7-dioxabicyclo[3.3.0]octane-3,8-dione Structure](https://image.chemsrc.com/caspic/346/475291-29-3.png)