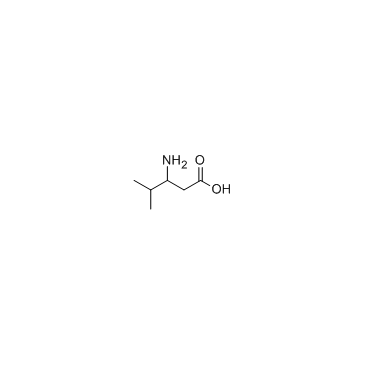
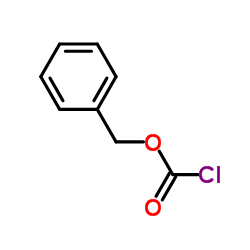
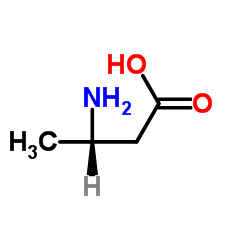
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

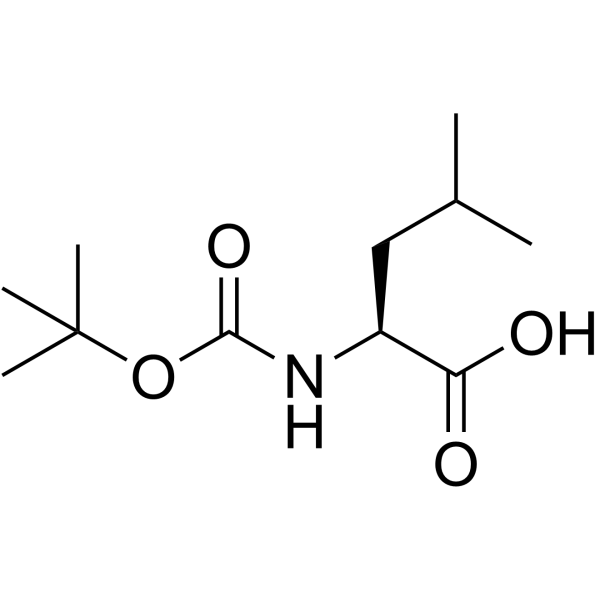
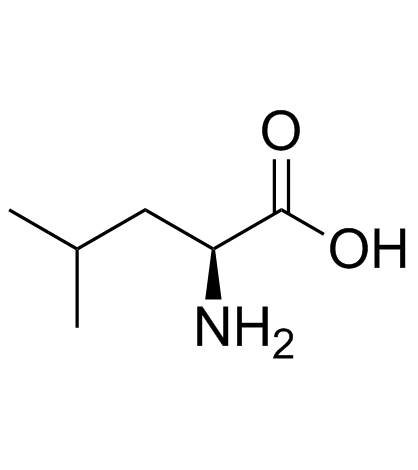


![(2S)-2-[(tert-butyloxycarbonyl)amino]-1-[(p-tolylsulfonyl)oxy]-4-methylpentane Structure](https://image.chemsrc.com/caspic/041/112157-30-9.png)
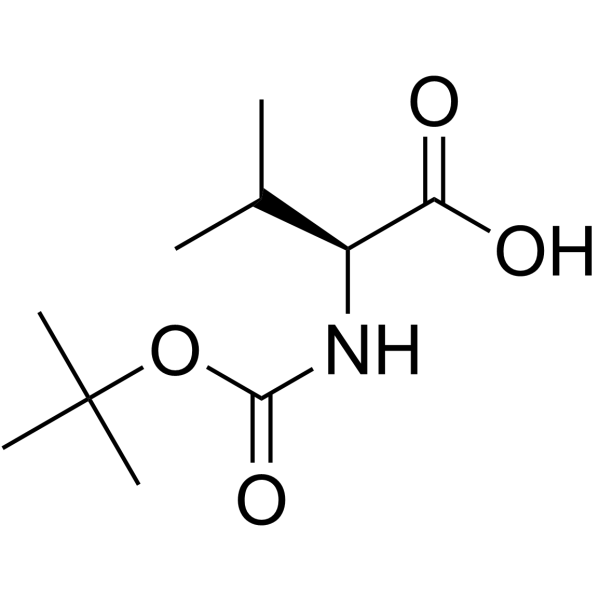
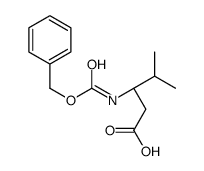
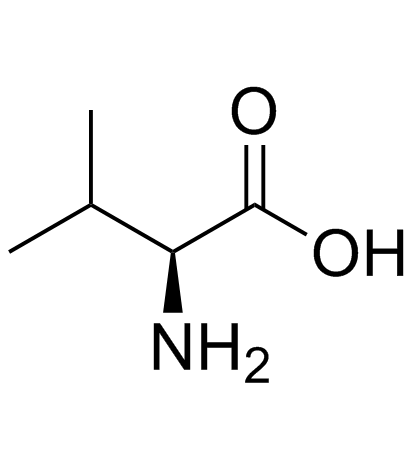
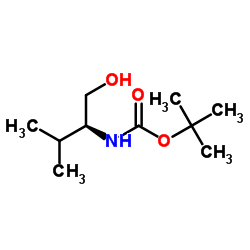
![(1S)-[2-methyl-1-[[[(4-methylphenyl)sulfonyl]oxy]methyl]propyl]carbamic acid 1,1-dimethylethyl ester Structure](https://image.chemsrc.com/caspic/108/122818-28-4.png)
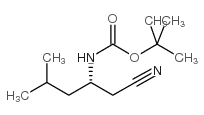
![Carbamic acid, [(1R)-1-(cyanomethyl)-2-methylpropyl]-, 1,1-dimethylethyl ester Structure](https://image.chemsrc.com/caspic/310/172695-23-7.png)