|
~82% |
|
~% |
|
~% |
|
~74% |
|
~% |
|
~91% |
|
~% |
|
~81% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~72% |
|
~% |
|
~72% |
|
~% |
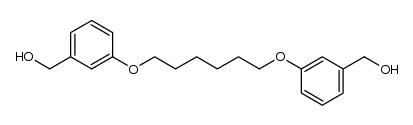
![1-(bromomethyl)-3-[6-[3-(bromomethyl)phenoxy]hexoxy]benzene Structure](https://image.chemsrc.com/caspic/476/560086-32-0.png)
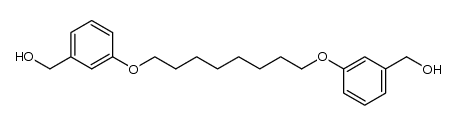
![1-(bromomethyl)-3-[8-[3-(bromomethyl)phenoxy]octoxy]benzene Structure](https://image.chemsrc.com/caspic/438/560086-33-1.png)
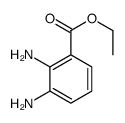
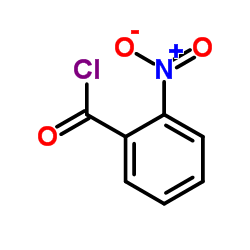
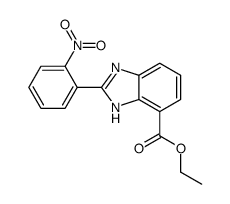
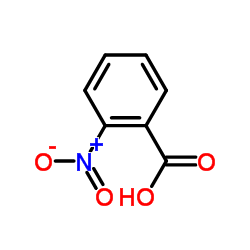

![1-(bromomethyl)-3-[10-[3-(bromomethyl)phenoxy]decoxy]benzene Structure](https://image.chemsrc.com/caspic/400/560086-34-2.png)
![1-(bromomethyl)-3-[2-[3-(bromomethyl)phenoxy]ethoxy]benzene Structure](https://image.chemsrc.com/caspic/062/560086-30-8.png)
![1-(bromomethyl)-3-[4-[3-(bromomethyl)phenoxy]butoxy]benzene Structure](https://image.chemsrc.com/caspic/024/560086-31-9.png)