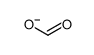
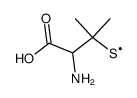
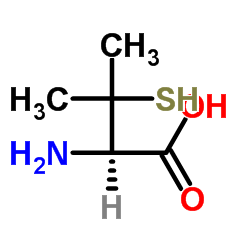
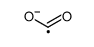
Reactivity of sulfur nucleophiles towards S-nitrosothiols
[Munro, Andrew P.; Williams, D. Lyn H. Journal of the Chemical Society. Perkin Transactions 2, 2000 , # 9 p. 1794 - 1797]
|
Cu (II)–Catalyzed Transformation of Benzylpenicillin Revisit...
[Kessler, David P.; Ghebre-Sellassie, Isaac; Knevel, Adelbert M.; Hem, Stanley L. Journal of the Chemical Society, Perkin Transactions 2: Physical Organic Chemistry (1972-1999), 1981 , p. 1247 - 1251]
|
Reaction of thiols and disulfides with phosphite radicals. A...
[Schaefer, K.; Asmus, K.-D. Journal of Physical Chemistry, 1981 , vol. 85, # 7 p. 852 - 855]
|
Reaction of ascorbic acid with S-nitrosothiols: clear eviden...
[Holmes, Anthony J.; Williams, D. Lyn H. Journal of the Chemical Society. Perkin Transactions 2, 2000 , # 8 p. 1639 - 1644]
|