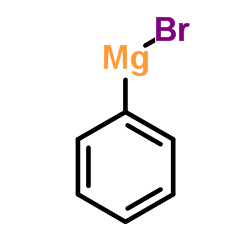
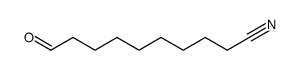
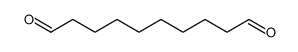
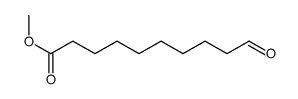
|
~84% |
|
~86% |
|
~91% |
|
~49% |
|
~% |
|
~93% |
|
~49% |
|
~74% |
|
~% |

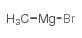
![1-[4-(2-hydroxypropan-2-yl)phenyl]ethanone Structure](https://image.chemsrc.com/caspic/011/54549-72-3.png)
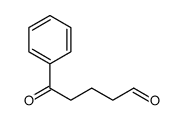
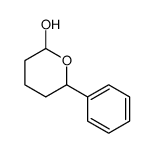
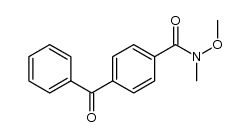
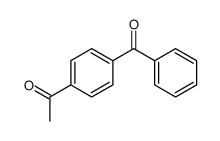
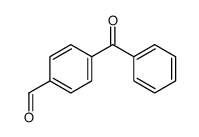
![4-[hydroxy(phenyl)methyl]benzaldehyde Structure](https://image.chemsrc.com/caspic/418/52010-95-4.png)