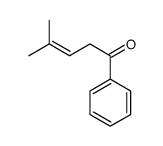
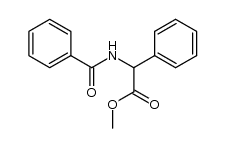
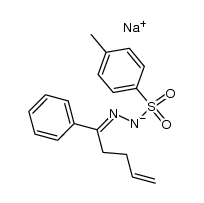
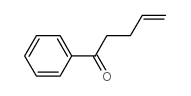
|
~% |
|
~% |
|
~20% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0%
Detail
|
|
~% |
|
~% |
|
~% |
|
~86% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~0% |
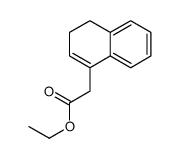

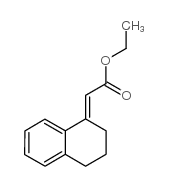

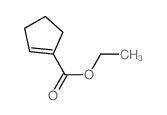

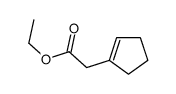
![3-phenyl-6-endo-ethyl-6-exo-methyl-1,2-diazabicyclo[3.1.0]hex-2-ene Structure](https://image.chemsrc.com/caspic/031/100189-08-0.png)

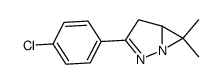
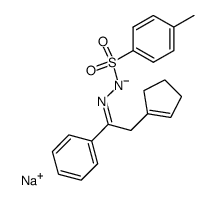
![2-phenyl-4a,5,6,7-tetrahydro-1H-cyclopenta[2,3]azirino[1,2-b]pyrazole Structure](https://image.chemsrc.com/caspic/239/73594-35-1.png)
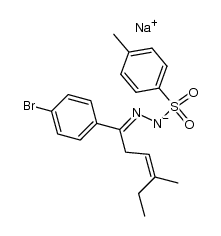
![3-(p-bromophenyl)-6-endo-ethyl-6-exo-methyl-1,2-diazabicyclo[3.1.0]hex-2-ene Structure](https://image.chemsrc.com/caspic/493/100189-09-1.png)

![3,6a-diphenyl-3,3a,4,5,6,6a-hexahydrocyclopenta[c]pyrazole Structure](https://image.chemsrc.com/caspic/264/87013-65-8.png)
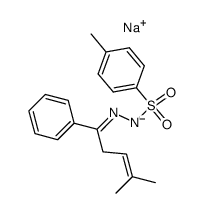
![3-phenyl-6,6'-dimethyl-1,2-diazabicyclo[3.1.0]hex-2-ene Structure](https://image.chemsrc.com/caspic/357/76620-31-0.png)
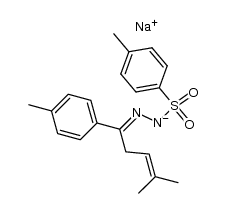

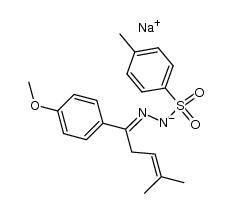
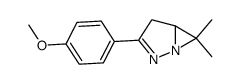
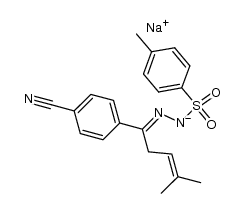
![3-(p-cyanophenyl)-6,6-dimethyl-1,2-diazabicyclo[3.1.0]hex-2-ene Structure](https://image.chemsrc.com/caspic/486/100189-14-8.png)
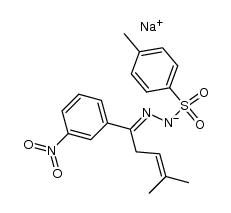
![3-(m-nitrophenyl)-6,6-dimethyl-1,2-diazabicyclo[3.1.0]hex-2-ene Structure](https://image.chemsrc.com/caspic/024/100189-13-7.png)

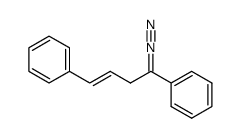
![(5R,6R)-3,6-diphenyl-1,2-diazabicyclo[3.1.0]hex-2-ene Structure](https://image.chemsrc.com/caspic/190/74457-37-7.png)
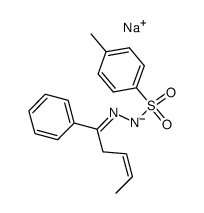
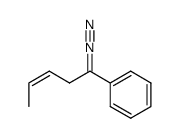
![exo-3-phenyl-6-methyl-1,2-diazabicyclo[3.1.0]hex-2-ene Structure](https://image.chemsrc.com/caspic/284/76633-73-3.png)