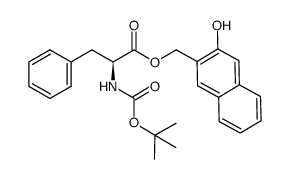
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![21-[(3-hydroxy-2-naphthalenyl)methoxy]progesterone Structure](https://image.chemsrc.com/caspic/418/1067231-68-8.png)
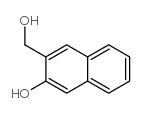
![O-[(3-hydroxy-2-naphthalenyl)methyl]estrone Structure](https://image.chemsrc.com/caspic/380/1067231-69-9.png)
![5'-O-[(3-hydroxy-2-naphthalenyl)methyl]-3'-O-methyl-3-methylthimidine Structure](https://image.chemsrc.com/caspic/015/1067231-70-2.png)