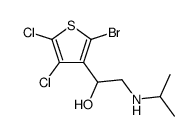
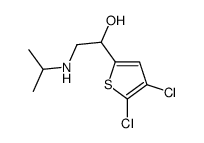
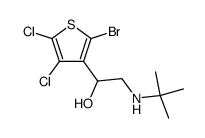
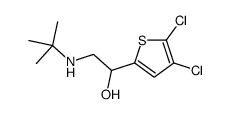
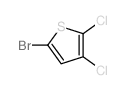
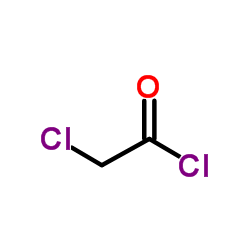
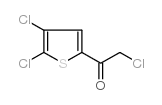
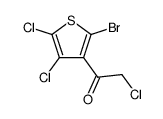
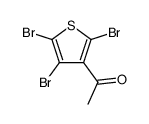
|
~53% |
|
~58% |
|
~% |
|
~59% |