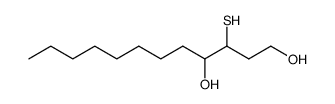
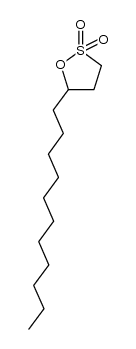
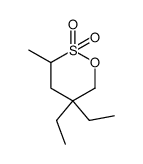
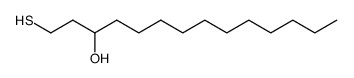
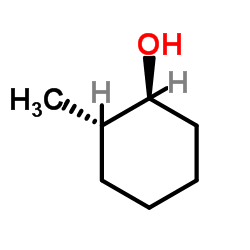
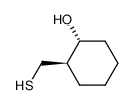
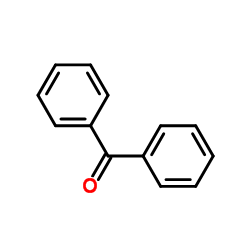
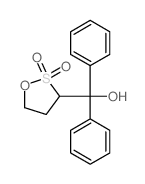
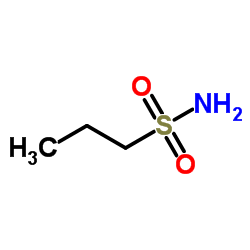
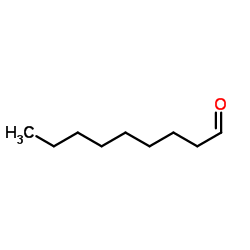
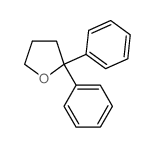
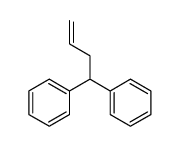
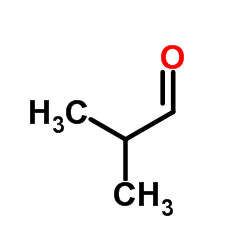
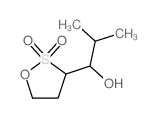
|
~24% |
|
~% |
|
~4% |
|
~3% |
|
~0% |
|
~% |
|
~5% |
|
~% |
|
~5% |
|
~% |
|
~16% |
|
~% |