|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![spiro[1-azabicyclo[2.2.2]octane-2,3'-pyrrolidine]-2'-one Structure](https://image.chemsrc.com/caspic/241/877606-85-4.png)
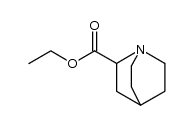
![1-Azabicyclo[2.2.1]heptane-2-carboxylicacid,ethylester(9CI) Structure](https://image.chemsrc.com/caspic/230/646055-79-0.png)
![Spiro[1-azabicyclo[2.2.1]heptane-2,3-pyrrolidine] (9CI) Structure](https://image.chemsrc.com/caspic/299/646055-82-5.png)
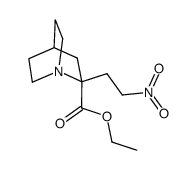
![ETHYL 2-(2-NITROETHYL)-1-AZABICYCLO[2.2.1]HEPTANE-2-CARBOXYLATE Structure](https://image.chemsrc.com/caspic/375/646055-80-3.png)
![1-Azabicyclo[2.2.1]heptane-7-carboxylicacid,ethylester(9CI) Structure](https://image.chemsrc.com/caspic/468/646055-95-0.png)
![Spiro[1-azabicyclo[2.2.1]heptane-7,3-pyrrolidin]-2-one (9CI) Structure](https://image.chemsrc.com/caspic/392/646055-97-2.png)
![Spiro[1-azabicyclo[2.2.1]heptane-7,3-pyrrolidine] (9CI) Structure](https://image.chemsrc.com/caspic/354/646055-98-3.png)
![ethyl 7-(2-nitroethyl)-1-aza-bicyclo[2.2.1]heptane-7-carboxylate Structure](https://image.chemsrc.com/caspic/430/646055-96-1.png)
![Spiro[1-azabicyclo[2.2.2]octane-2,3-pyrrolidine] (9CI) Structure](https://image.chemsrc.com/caspic/168/646055-90-5.png)
![Spiro[1-azabicyclo[2.2.1]heptane-2,3-pyrrolidin]-2-one (9CI) Structure](https://image.chemsrc.com/caspic/337/646055-81-4.png)