|
~% |
|
~% |
|
~78% |
|
~% |
|
~% |

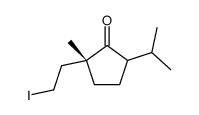

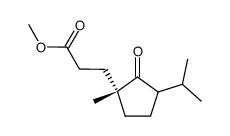
![3-[(1R)-1-methyl-2-oxo-3-propan-2-ylcyclopentyl]propanoic acid Structure](https://image.chemsrc.com/caspic/134/868615-50-3.png)