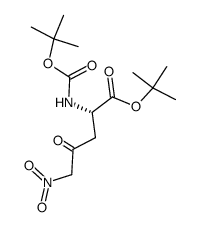
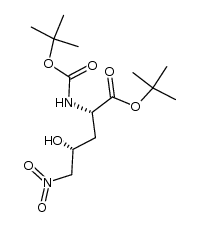
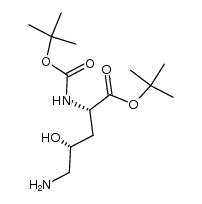
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

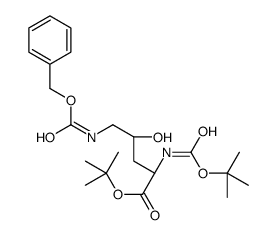
![1-tert-butyl (S)-2-[N-(tert-butoxycarbonyl)amino]-4-oxobutanoate Structure](https://image.chemsrc.com/caspic/092/81323-59-3.png)
![2-Methyl-2-propanyl N-{[(2-methyl-2-propanyl)oxy]carbonyl}-L-homoserinate Structure](https://image.chemsrc.com/caspic/130/81323-58-2.png)