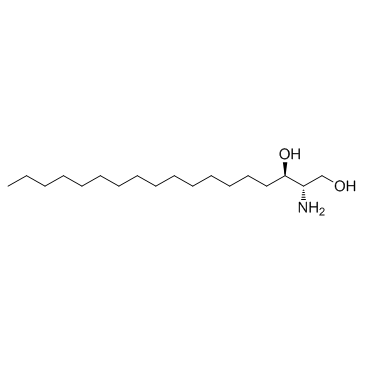
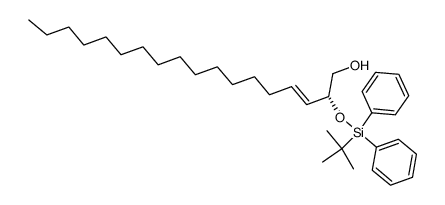
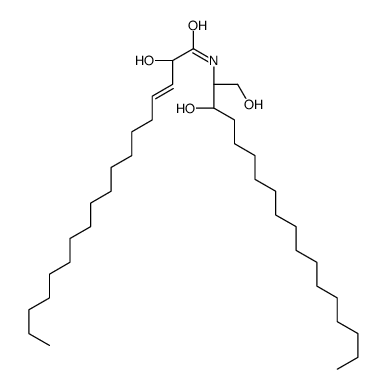
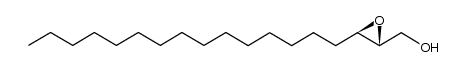
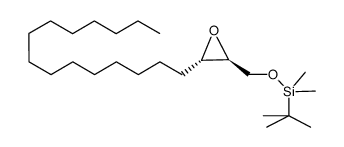
|
~95% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![N-[(1S,2R)-2-Hydroxy-1-(hydroxyMethyl)heptadecyl]carbamic Acid 1,1-Dimethylethyl Ester Structure](https://image.chemsrc.com/caspic/166/140408-14-6.png)