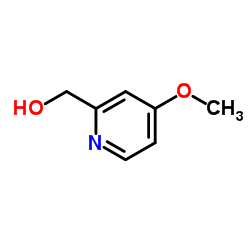
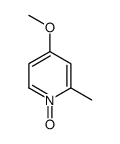
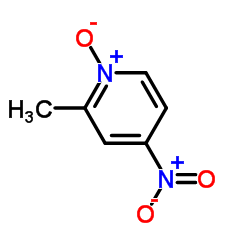
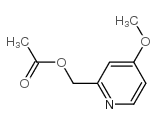
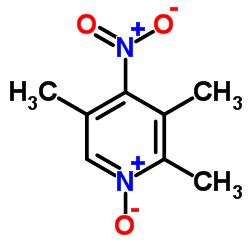
|
~% |
|
~86% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~89% |
|
~90% |
|
~94% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
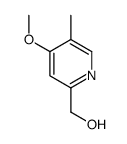
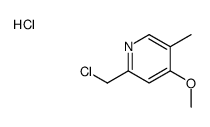

![2,2-difluoro-6-[(4-methoxy-3-methylpyridin-2-yl)methylsulfinyl]-5H-[1,3]dioxolo[4,5-f]benzimidazole Structure](https://image.chemsrc.com/caspic/191/97966-86-4.png)
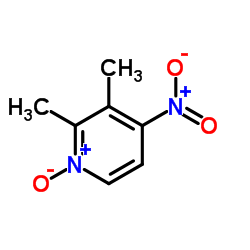
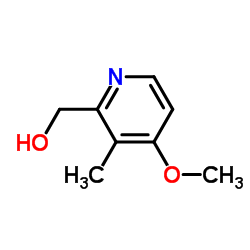
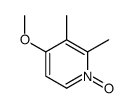
![2,2-difluoro-6-mercapto-5H-[1,3]dioxolo-[4,5-f]benzimidazole Structure](https://image.chemsrc.com/caspic/211/97967-01-6.png)
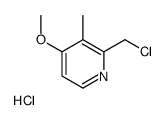
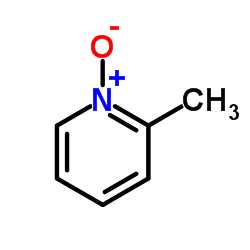
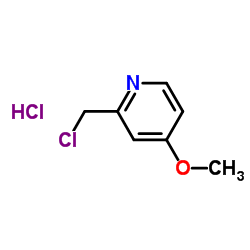

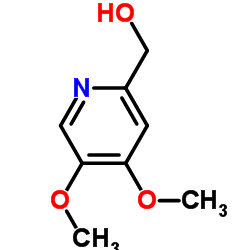
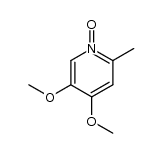
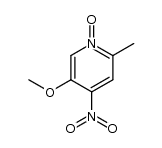
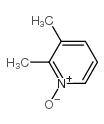
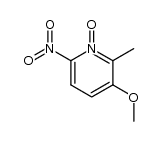
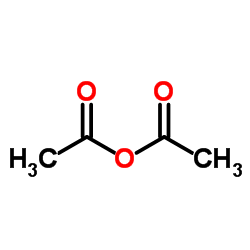
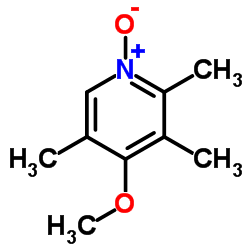


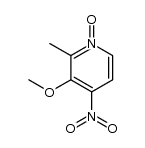
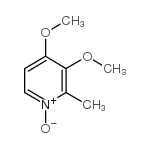
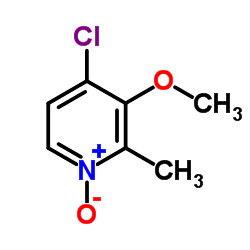
![2-[(4-methoxy-pyridin-2-yl)methylthio]-5-(1,1,2,2-tetrafluoroethoxy)-1H-benzimidazole Structure](https://image.chemsrc.com/caspic/390/97964-15-3.png)
![2-[(4-methoxypyridin-2-yl)methylsulfinyl]-6-(1,1,2,2-tetrafluoroethoxy)-1H-benzimidazole Structure](https://image.chemsrc.com/caspic/032/97963-96-7.png)