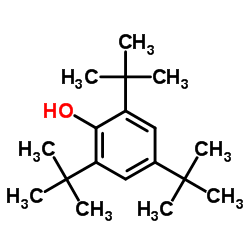
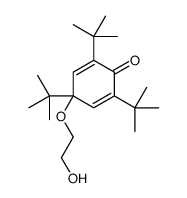
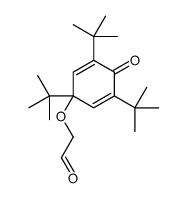
|
~16% |
|
~72% |
|
~76% |
|
~53% |
|
~71% |
|
~31% |
|
~86% |
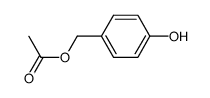
![[1-(2-hydroxyethoxy)-4-oxocyclohexa-2,5-dien-1-yl]methyl acetate Structure](https://image.chemsrc.com/caspic/143/941282-79-7.png)

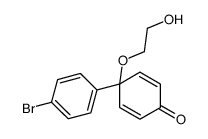
![2-[1-(4-bromophenyl)-4-oxocyclohexa-2,5-dien-1-yl]oxyacetaldehyde Structure](https://image.chemsrc.com/caspic/150/881181-53-9.png)
![[4-oxo-1-(2-oxoethoxy)cyclohexa-2,5-dien-1-yl]methyl acetate Structure](https://image.chemsrc.com/caspic/064/881181-55-1.png)
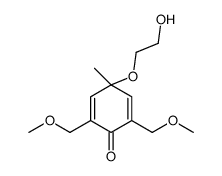
![2-[3,5-bis(methoxymethyl)-1-methyl-4-oxocyclohexa-2,5-dien-1-yl]oxyacetaldehyde Structure](https://image.chemsrc.com/caspic/433/881181-63-1.png)