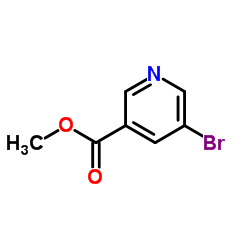
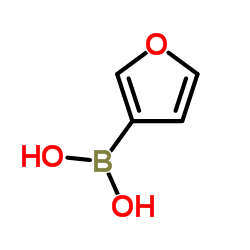
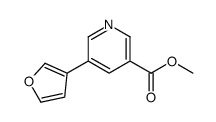
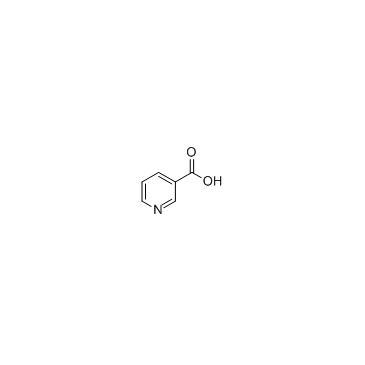
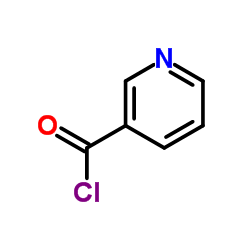
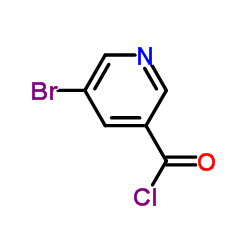
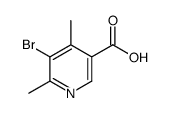
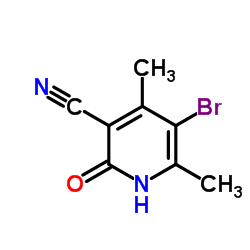
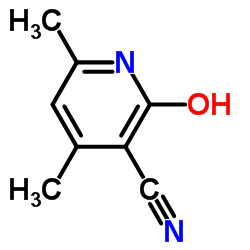
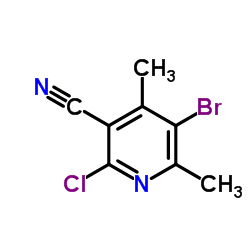
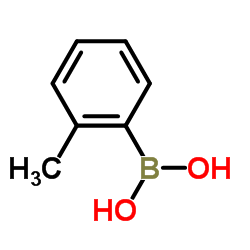
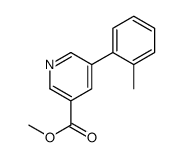
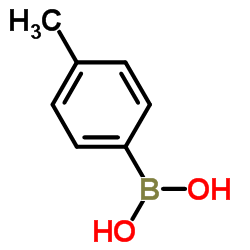
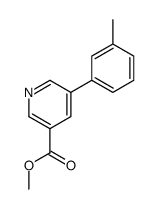
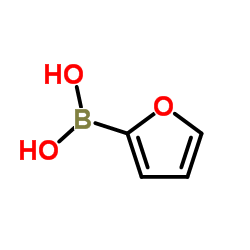
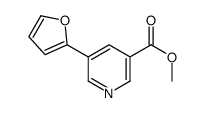
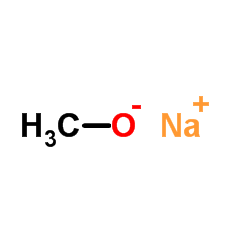
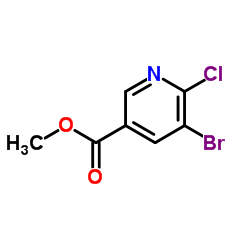
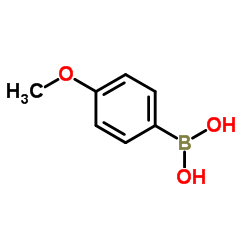
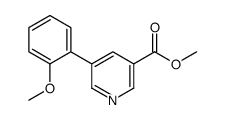
|
~82% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~95% |
|
~80% |
|
~% |
|
~% |
|
~% |
|
~96% |
|
~% |
|
~% |
|
~% |
|
~86% |
|
~96% |
|
~61% |
|
~% |
|
~% |
|
~70% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~95% |
|
~% |
|
~% |
|
~% |