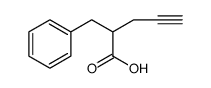
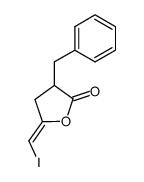
|
~89% |
|
~% |
|
~% |
|
~30% |
|
~% |
|
~% |
|
~% |
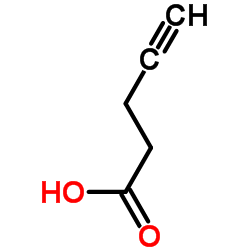
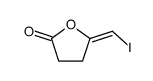

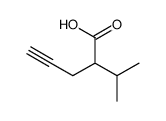
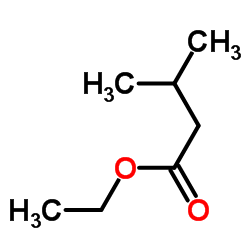
![3-benzyl-5(E)-[3-(trimethylsilyl)prop-2-ynylidene]tetrahydro-2-furanone Structure](https://image.chemsrc.com/caspic/143/103437-64-5.png)