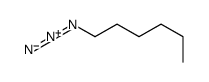
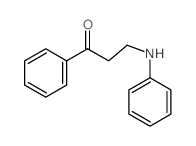
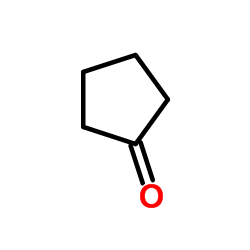
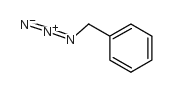
|
~59% |
|
~81% |
|
~73% |
|
~5% |
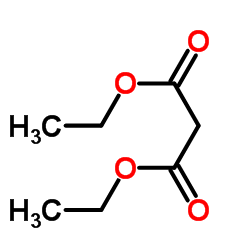
![Propanedioicacid, 2-[(phenylamino)methyl]-, 1,3-diethyl ester Structure](https://image.chemsrc.com/caspic/467/77779-37-4.png)