|
~87% |
|
~76% |
|
~75% |
|
~56% |
|
~83% |
|
~59% |
|
~77% |
|
~87% |
|
~55% |
|
~62% |
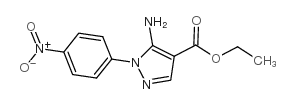
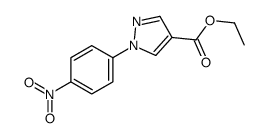
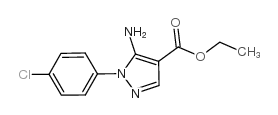
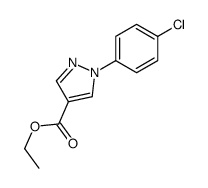
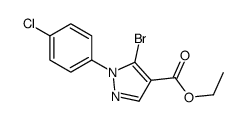
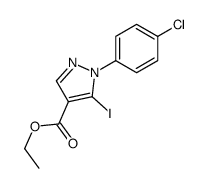
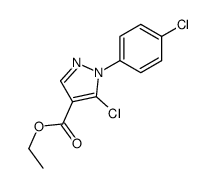
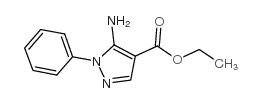
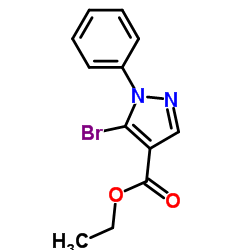
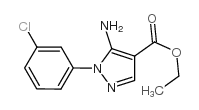

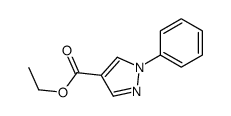
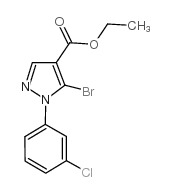

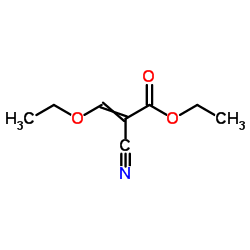
![5-AMINO-1-[5-(FLUOROMETHYL)PHENYL]-1H-PYRAZOLE-4-CARBOXYLIC ACID ETHYL ESTER Structure](https://image.chemsrc.com/caspic/333/110821-29-9.png)