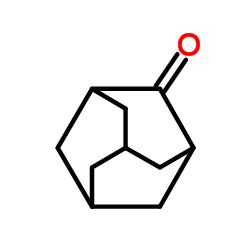
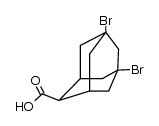
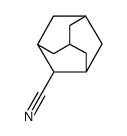
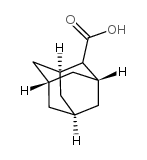
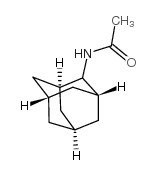
|
~99% |
|
~% |
|
~64% |
|
~% |
|
~% |
|
~90% |
|
~86% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~74% |
![(4-hydroxymethyl-benzo[1,3]dioxol-5-yl)methanol Structure](https://image.chemsrc.com/caspic/227/1077-75-4.png)
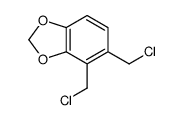
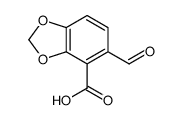

![2-[4-(cyanomethyl)-1,3-benzodioxol-5-yl]acetonitrile Structure](https://image.chemsrc.com/caspic/300/871587-53-0.png)
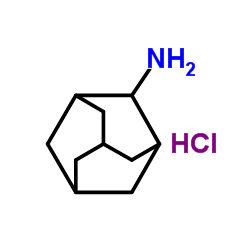
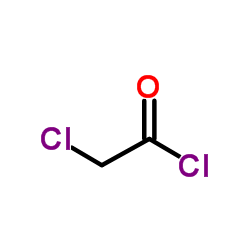

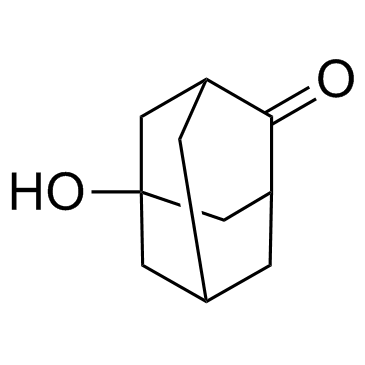

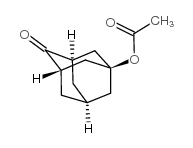
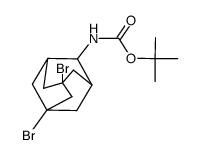
![9-amino-7-methylidenebicyclo[3.3.1]nonan-3-one Structure](https://image.chemsrc.com/caspic/211/918830-96-3.png)