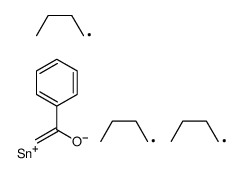
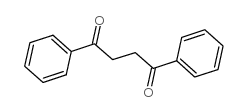
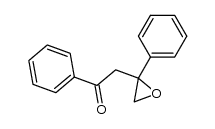
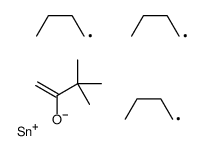
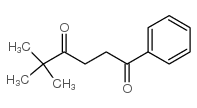
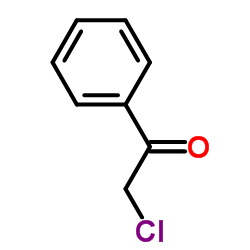
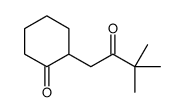
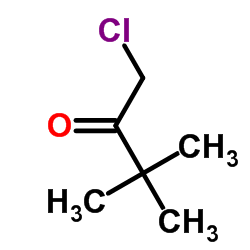
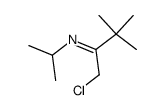
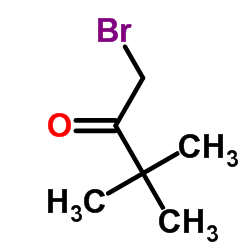
|
~72% |
|
~4%
Detail
|
|
~44% |
|
~% |
|
~21% |
|
~77% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |