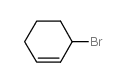
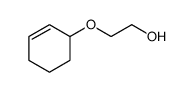
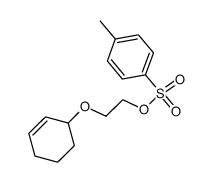
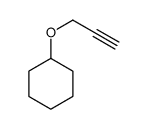
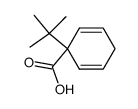
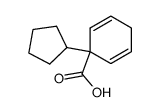
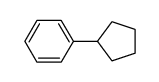
|
~% |
|
~53% |
|
~% |
|
~% |
|
~12% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~58% |
|
~84% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~8% |
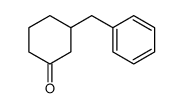
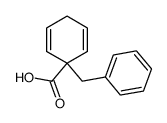
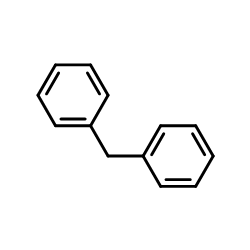
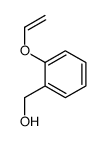


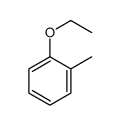
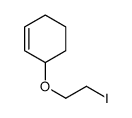
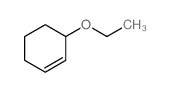
![2-Oxabicyclo[4.3.0]nonane Structure](https://image.chemsrc.com/caspic/479/10198-29-5.png)