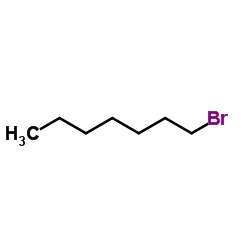
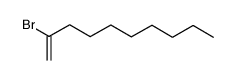
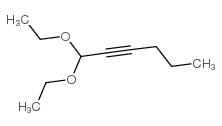
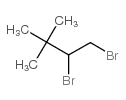
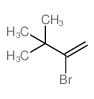
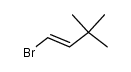
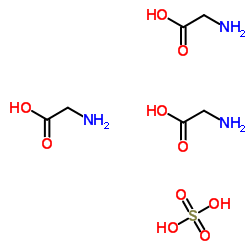
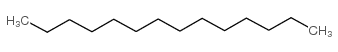
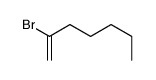
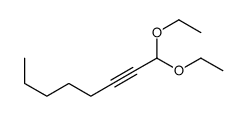
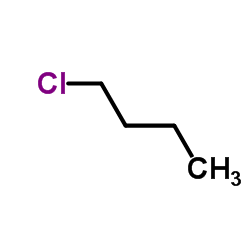
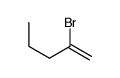
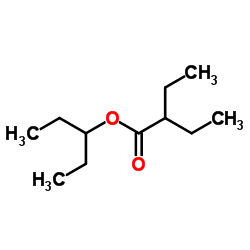
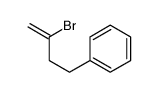
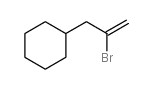
|
~% |
|
~% |
|
~46% |
|
~% |
|
~43% |
|
~% |
|
~51% |
|
~78% |
|
~59% |
|
~56% |