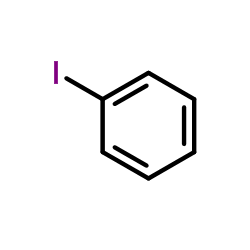
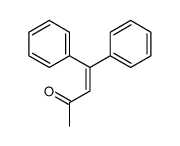
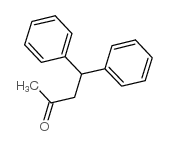
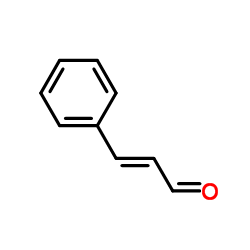
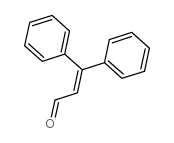
|
~76% |
|
~% |
|
~55% |
|
~99% |