|
~77% |
|
~% |
|
~68% |
|
~66% |
|
~% |
|
~81% |
|
~% |
|
~76% |
|
~% |
|
~79% |
|
~% |
|
~79% |
|
~% |
|
~79% |
|
~% |
|
~83% |
|
~80% |
|
~% |
|
~83% |
|
~% |
|
~81% |
|
~87% |
|
~% |
|
~83% |
|
~% |
|
~78% |
|
~72% |
|
~% |
|
~73% |
|
~80% |
|
~78% |
|
~82% |
|
~80% |
|
~72% |
|
~% |
|
~90% |
|
~84% |
|
~% |
|
~% |
|
~71% |
|
~% |
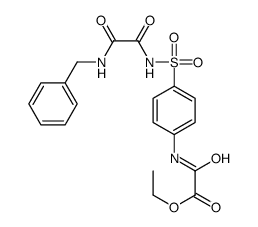
![N-benzyl-N'-[4-[[2-(hydroxyamino)-2-oxoacetyl]amino]phenyl]sulfonyloxamide Structure](https://image.chemsrc.com/caspic/016/81717-48-8.png)
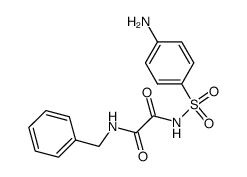
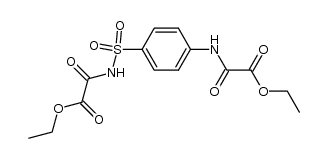
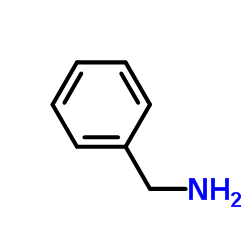
![N-benzyl-N'-[4-[[2-(benzylamino)-2-oxoacetyl]sulfamoyl]phenyl]oxamide Structure](https://image.chemsrc.com/caspic/468/81717-27-3.png)
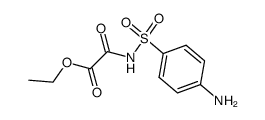
![ethyl 2-[4-[[2-(2-methylpropylamino)-2-oxoacetyl]sulfamoyl]anilino]-2-oxoacetate Structure](https://image.chemsrc.com/caspic/206/81717-21-7.png)
![N'-[4-[[2-(hydroxyamino)-2-oxoacetyl]amino]phenyl]sulfonyl-N'-(2-methylpropyl)oxamide Structure](https://image.chemsrc.com/caspic/499/81717-31-9.png)
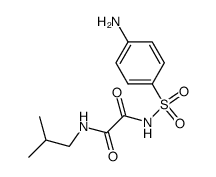
![ethyl [4-[(2-hydroxyethylcarbamoylformyl)sulfamoyl]phenyl]carbamoylfor mate Structure](https://image.chemsrc.com/caspic/137/81717-18-2.png)
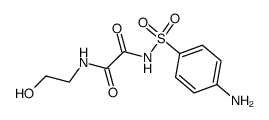
![N'-[4-[[2-(2-hydroxyethylamino)-2-oxoacetyl]sulfamoyl]phenyl]-N-methyloxamide Structure](https://image.chemsrc.com/caspic/413/81717-33-1.png)
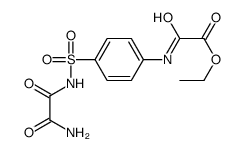
![N-[4-[(hydroxycarbamoylformyl)amino]phenyl]sulfonyloxamide Structure](https://image.chemsrc.com/caspic/478/81717-49-9.png)
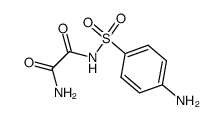
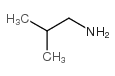
![N-(2-hydroxyethyl)-N'-[4-[[2-(2-methylpropylamino)-2-oxoacetyl]amino]phenyl]sulfonyloxamide Structure](https://image.chemsrc.com/caspic/375/81717-34-2.png)
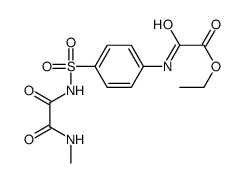
![N'-[4-[methyl(oxamoyl)sulfamoyl]phenyl]oxamide Structure](https://image.chemsrc.com/caspic/223/81717-38-6.png)
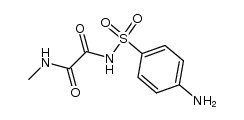

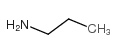
![N'-methyl-N'-[4-[[2-oxo-2-(propylamino)acetyl]amino]phenyl]sulfonyloxamide Structure](https://image.chemsrc.com/caspic/185/81717-39-7.png)
![N'-[4-[[2-(methylamino)-2-oxoacetyl]sulfamoyl]phenyl]-N'-(2-methylpropyl)oxamide Structure](https://image.chemsrc.com/caspic/282/81717-41-1.png)
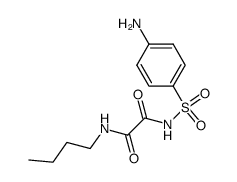
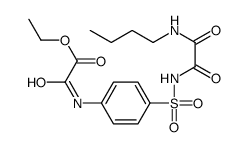
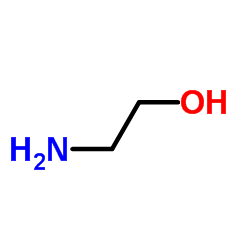
![N'-[4-[[2-(2-hydroxyethylamino)-2-oxoacetyl]amino]phenyl]sulfonyl-N'-methyloxamide Structure](https://image.chemsrc.com/caspic/244/81717-42-2.png)
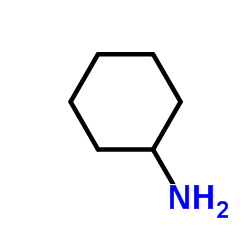
![N'-[4-[[2-(cyclohexylamino)-2-oxoacetyl]amino]phenyl]sulfonyl-N-methyloxamide Structure](https://image.chemsrc.com/caspic/206/81717-43-3.png)
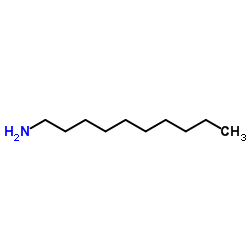
![N'-[4-[[2-(decylamino)-2-oxoacetyl]amino]phenyl]sulfonyl-N'-methyloxamide Structure](https://image.chemsrc.com/caspic/130/81717-45-5.png)
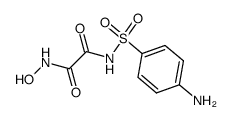
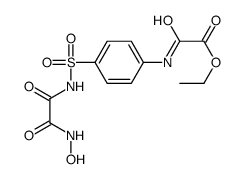
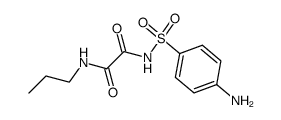
![ethyl [4-[(propylcarbamoylformyl)sulfamoyl]phenyl]carbamoylformate Structure](https://image.chemsrc.com/caspic/099/81717-19-3.png)
![N'-benzyl-N'-[4-[[2-(methylamino)-2-oxoacetyl]sulfamoyl]phenyl]oxamide Structure](https://image.chemsrc.com/caspic/092/81717-46-6.png)
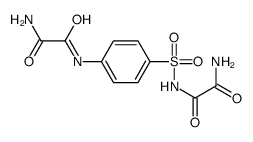
![N'-[4-[[2-(hydroxyamino)-2-oxoacetyl]amino]phenyl]sulfonyl-N-propyloxamide Structure](https://image.chemsrc.com/caspic/054/81717-47-7.png)