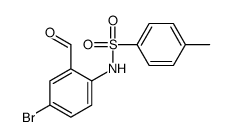
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
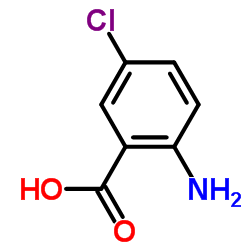
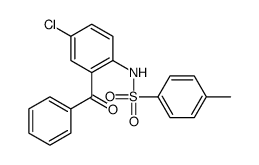
![5-chloro-2-[(4-methylphenyl)sulfonylamino]benzoic acid Structure](https://image.chemsrc.com/caspic/261/897-82-5.png)
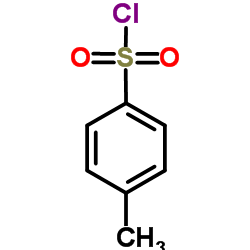
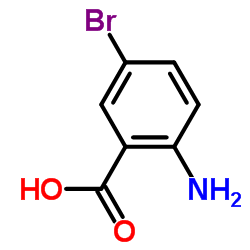

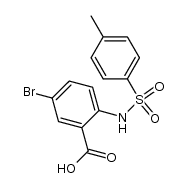
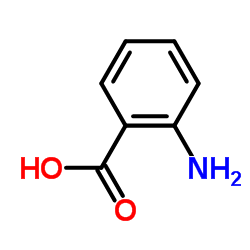
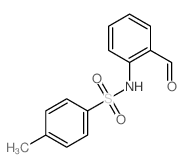
![2-[(4-methylphenyl)sulfonylamino]benzoic acid Structure](https://image.chemsrc.com/caspic/437/6311-23-5.png)