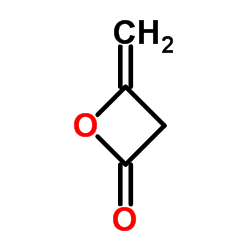
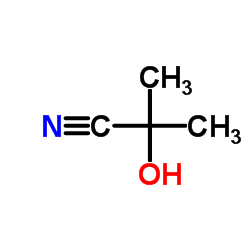
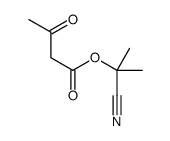
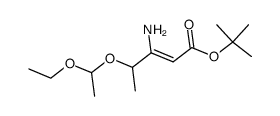
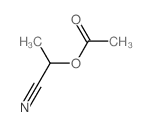
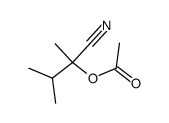
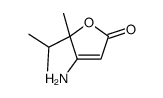
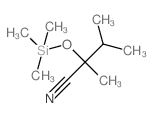
|
~90% |
|
~70% |
|
~6% |
|
~60% |
|
~% |
|
~87% |