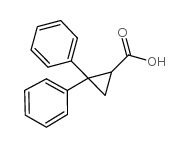
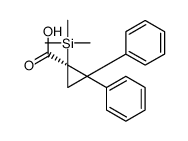
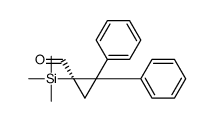
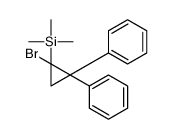
|
~89% |
|
~% |
|
~% |
|
~78% |
|
~% |
|
~% |
|
~% |
|
~58% |
|
~% |

![[(1R)-2,2-diphenyl-1-trimethylsilylcyclopropyl]methanol Structure](https://image.chemsrc.com/caspic/334/88035-73-8.png)