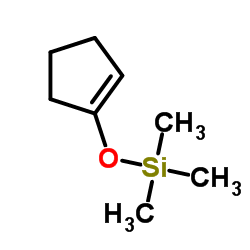
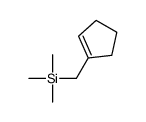
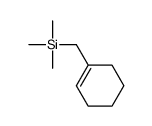
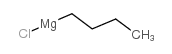
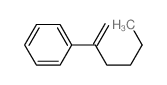
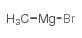|
~87% |
|
~84% |
|
~73% |
|
~72% |
|
~% |
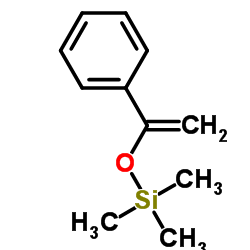
![[(Trimethylsilyl)Methyl]Magnesium Chloride Structure](https://image.chemsrc.com/caspic/432/13170-43-9.png)