|
~83% |
|
~81% |
|
~% |
|
~% |
|
~% |
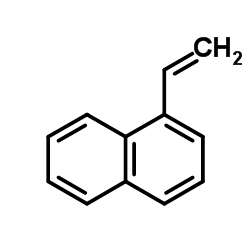
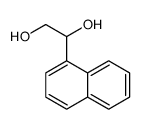

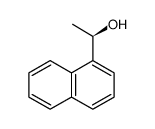
![(+)-1-[(1S),2-dihydroxyethyl]naphthalene Structure](https://image.chemsrc.com/caspic/009/209622-45-7.png)