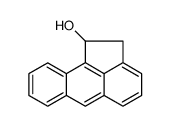
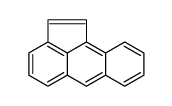
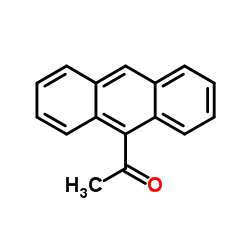
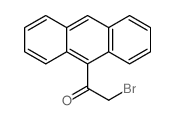
|
~94% |
|
~% |
|
~% |
|
~% |
|
~95% |
|
~% |
|
~% |
|
~56% |
|
~61% |
|
~% |