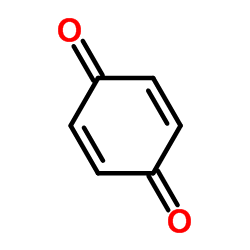
|
~42% |
|
~% |
|
~57% |
|
~% |
|
~% |
|
~% |
|
~% |
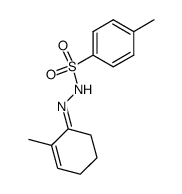

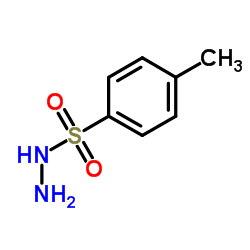


![3,6-dimethoxy-11-methyl-1,8,9,10-tetrahydrotricyclo[6.2.2.02,7]dodeca-3,9-diene Structure](https://image.chemsrc.com/caspic/217/79997-75-4.png)