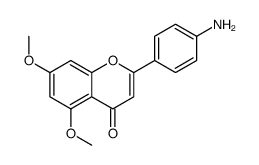
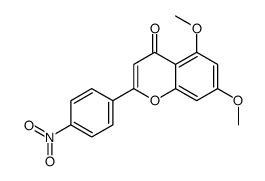
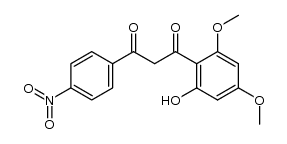
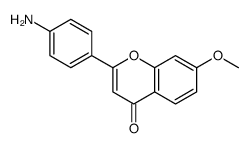
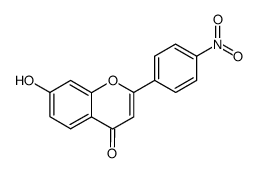
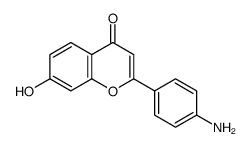
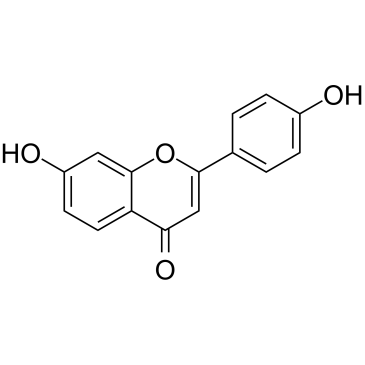
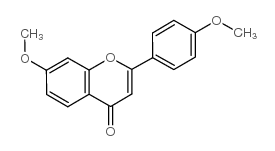
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

![1-(1-hydroxy-[2]naphthyl)-3-(4-nitro-phenyl)-propane-1,3-dione Structure](https://image.chemsrc.com/caspic/020/412018-11-2.png)
![2-(4-nitrophenyl)benzo[h]chromen-4-one Structure](https://image.chemsrc.com/caspic/022/71601-18-8.png)