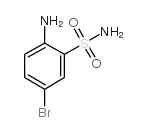
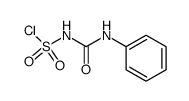
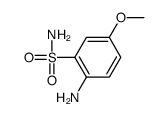
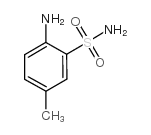
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~40% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![N-[(4-methylphenyl)carbamoyl]sulfamoyl chloride Structure](https://image.chemsrc.com/caspic/258/380885-39-2.png)
![7-METHYL-1,1-DIOXO-1,4-DIHYDRO-2H-1LAMBDA6-BENZO[1,2,4]THIADIAZIN-3-ONE Structure](https://image.chemsrc.com/caspic/480/71254-63-2.png)
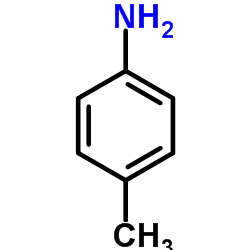
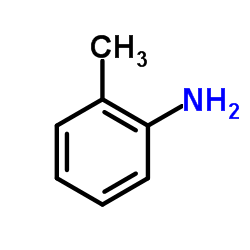
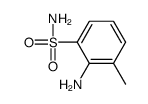
![5-methyl-1,1-dioxo-1,4-dihydro-2H-1λ6-benzo[1,2,4]thiadiazin-3-one Structure](https://image.chemsrc.com/caspic/442/71254-64-3.png)
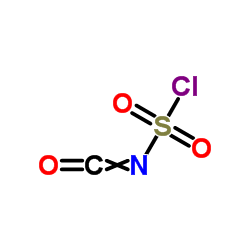
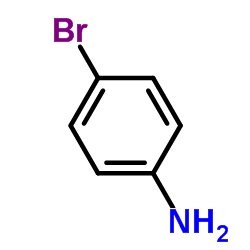
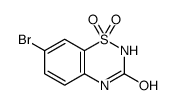
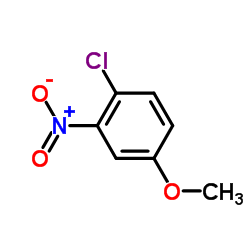
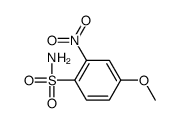
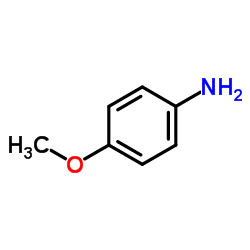
![7-METHOXY-1,1-DIOXO-1,4-DIHYDRO-2H-1LAMBDA6-BENZO[1,2,4]THIADIAZIN-3-ONE Structure](https://image.chemsrc.com/caspic/328/71254-67-6.png)