|
~90% |
|
~% |
|
~91% |
|
~90% |
|
~% |
|
~77% |
|
~% |
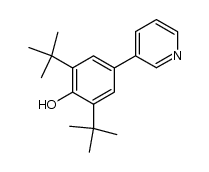
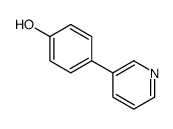
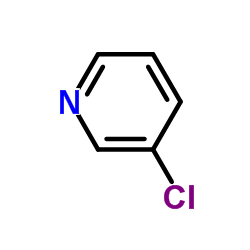
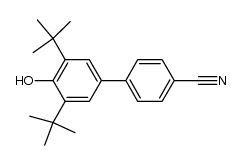


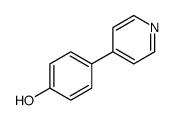

![4'-[(4-Chlorophenyl)sulfonyl]-3,5-di-tert-butyl-[1,1'-biphenyl]-4-ol Structure](https://image.chemsrc.com/caspic/302/139225-66-4.png)
![4'-((4-Chlorophenyl)sulfonyl)-[1,1'-biphenyl]-4-ol Structure](https://image.chemsrc.com/caspic/449/30809-75-7.png)
