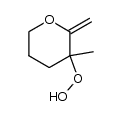
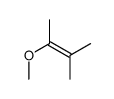
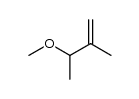
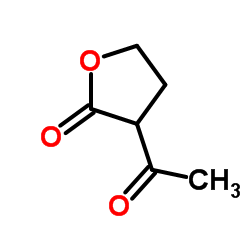
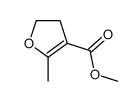
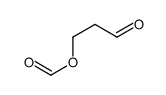
|
~86% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~19% |
|
~% |
|
~% |
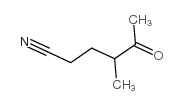
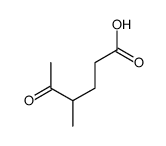
![1,6-Dimethyl-2,7,8-trioxabicyclo[4.2.0]octane Structure](https://image.chemsrc.com/caspic/188/133969-07-0.png)