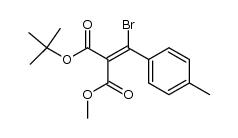
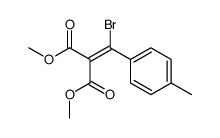
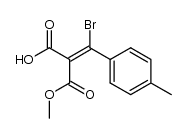
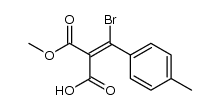
|
~0% |
|
~74% |
|
~% |
|
~% |
|
~% |
![dimethyl 2-[bromo-(4-nitrophenyl)methylidene]propanedioate Structure](https://image.chemsrc.com/caspic/183/103883-91-6.png)

![dimethyl 2-[(4-methylphenyl)methylidene]propanedioate Structure](https://image.chemsrc.com/caspic/266/59832-45-0.png)
![dimethyl 2-[(4-methylphenyl)methyl]propanedioate Structure](https://image.chemsrc.com/caspic/323/49769-82-6.png)