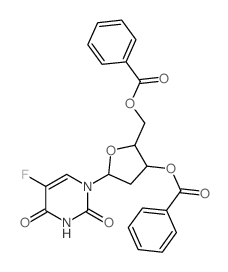
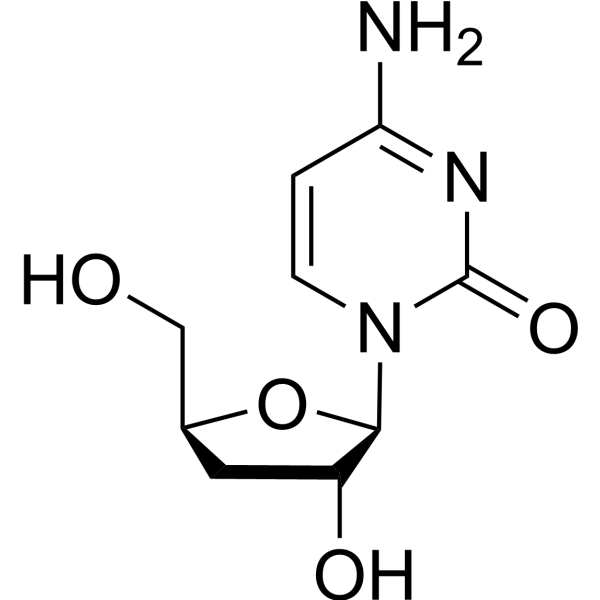
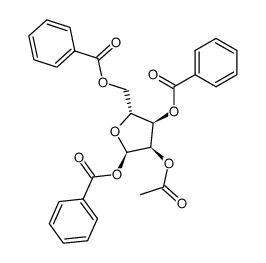
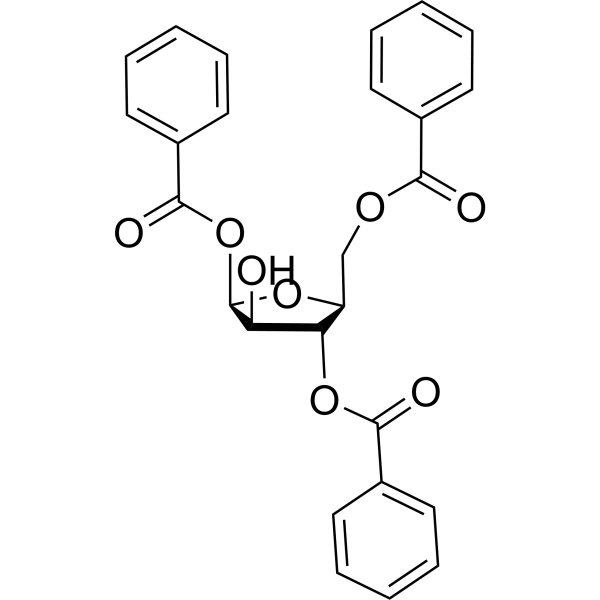
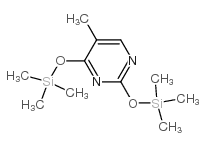
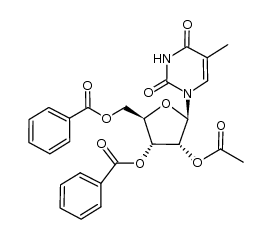
|
~% |
|
~53% |
|
~78% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~32% |
|
~% |
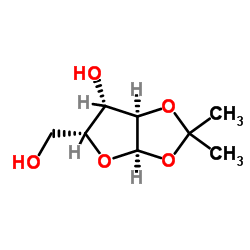

![3',5'-di-O-benzoyl-2'-O-[3-(trifluoromethyl)benzoyl]-5-fluorouridine Structure](https://image.chemsrc.com/caspic/473/182004-60-0.png)