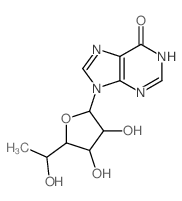
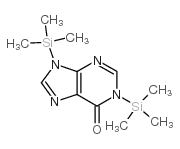
|
~% |
|
~% |
|
~% |
|
~% |
|
~68% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![9-[6'-deoxy-2',3',5'-tris-O-(p-nitrobenzoyl)-β-D-allofuranosyl]-6-thiopurine Structure](https://image.chemsrc.com/caspic/017/85421-87-0.png)
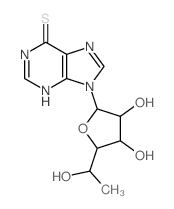
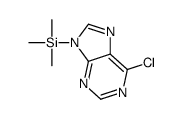
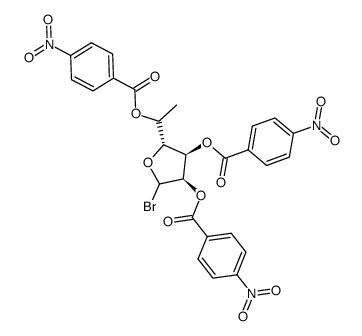
![9-[6'-deoxy-2',3',5'-tris-O-(p-nitrobenzoyl)-β-D-allofuranosyl]hypoxanthine Structure](https://image.chemsrc.com/caspic/255/85421-81-4.png)