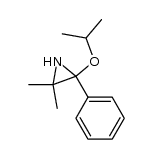
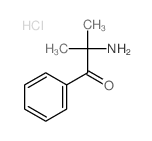
|
~33% |
|
~31% |
|
~% |
|
~% |
|
~76% |
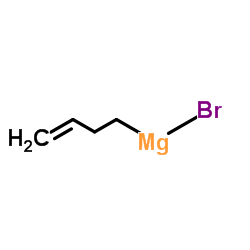
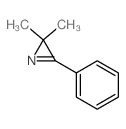
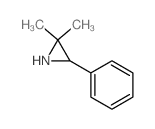
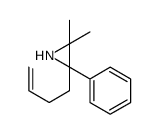
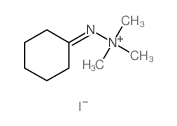
![6-but-3-enyl-7-azabicyclo[4.1.0]heptane Structure](https://image.chemsrc.com/caspic/162/61686-93-9.png)
![4-(7-azabicyclo[4.1.0]heptan-6-yl)butan-1-ol Structure](https://image.chemsrc.com/caspic/038/61686-96-2.png)