|
~60% |
|
~58% |
|
~% |
|
~% |
|
~% |
|
~0%
Detail
|
|
~63% |

![4-Thiazolecarboxamide, 2-[5-(hydroxymethyl)-2-furanyl] Structure](https://image.chemsrc.com/caspic/031/60084-14-2.png)

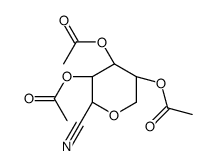

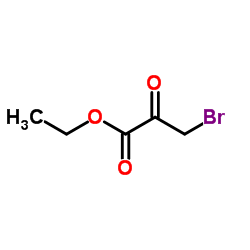
![ethyl 2-[5-(ethoxymethyl)furan-2-yl]-1,3-thiazole-4-carboxylate Structure](https://image.chemsrc.com/caspic/069/60084-13-1.png)
![2-[5-(ethoxymethyl)furan-2-yl]-1,3-thiazole-4-carboxamide Structure](https://image.chemsrc.com/caspic/493/60084-15-3.png)