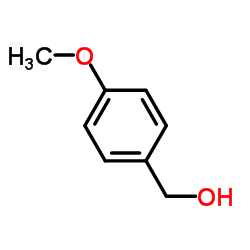
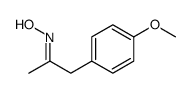
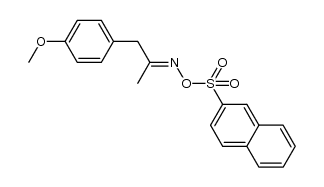
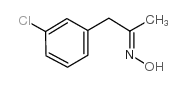
|
~86% |
|
~% |
|
~% |
|
~% |
|
~24% |
|
~98% |
|
~97% |
|
~97% |
|
~92% |
|
~99% |
|
~99% |
|
~99% |
|
~% |
|
~% |
|
~60% |
|
~% |
|
~% |
|
~% |
|
~90% |
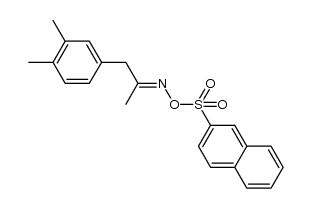
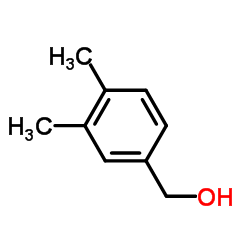
![N-[(3,4-dimethylphenyl)methyl]acetamide Structure](https://image.chemsrc.com/caspic/090/65609-15-6.png)
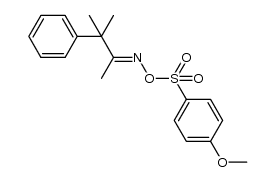
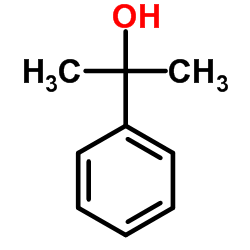
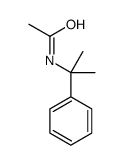
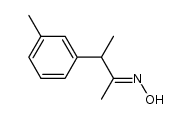
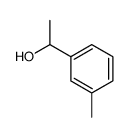

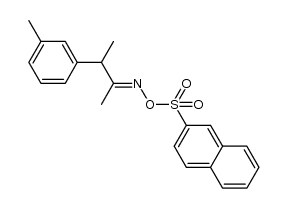

![N-[(4-cyanophenyl)methyl]acetamide Structure](https://image.chemsrc.com/caspic/177/98088-12-1.png)
![ACETAMIDE, N-[(4-CHLOROPHENYL)METHYL] Structure](https://image.chemsrc.com/caspic/159/57058-33-0.png)
![ACETAMIDE, N-[(4-METHYLPHENYL)METHYL] Structure](https://image.chemsrc.com/caspic/153/25079-96-3.png)








![ACETAMIDE, N-[(4-METHOXYPHENYL)METHYL] Structure](https://image.chemsrc.com/caspic/440/35103-34-5.png)