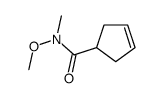
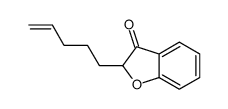
|
~% |
|
~% |
|
~% |
|
~96% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~96% |
|
~% |
|
~% |
|
~% |
|
~87% |
|
~% |
|
~% |
|
~% |
|
~92% |
|
~% |

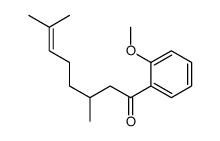
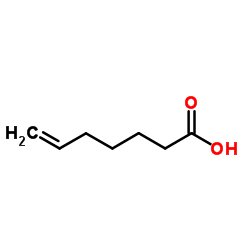

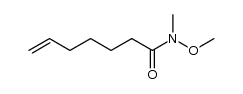
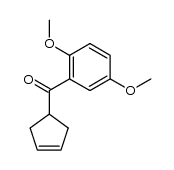
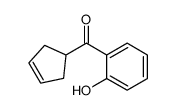
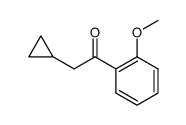
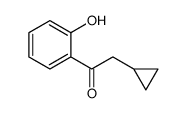
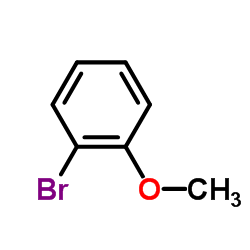
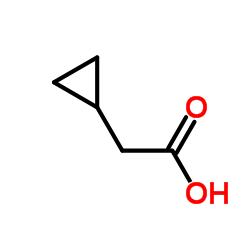
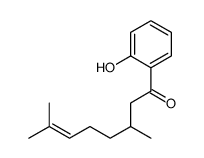
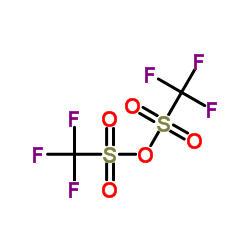
![[2-(2-cyclopropylacetyl)phenyl] trifluoromethanesulfonate Structure](https://image.chemsrc.com/caspic/231/646522-76-1.png)
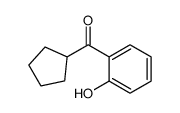
![[2-(cyclopentanecarbonyl)phenyl] trifluoromethanesulfonate Structure](https://image.chemsrc.com/caspic/117/646522-79-4.png)