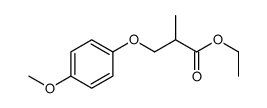
|
~74% |
|
~88% |
|
~% |
|
~85% |
|
~68% |
|
~94% |
|
~97% |

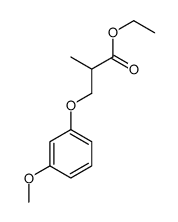
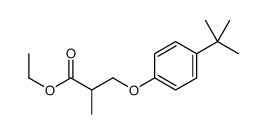


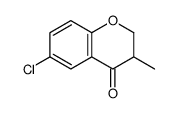
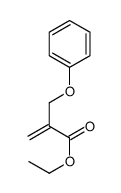
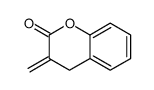
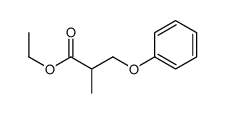
![ethyl 2-[(4-methoxyphenoxy)methyl]acrylate Structure](https://image.chemsrc.com/caspic/396/313674-73-6.png)