|
~16% |
|
~% |
|
~% |
|
~30% |
|
~% |
|
~12% |
|
~45% |
|
~6% |
|
~9% |
|
~25% |
|
~39% |
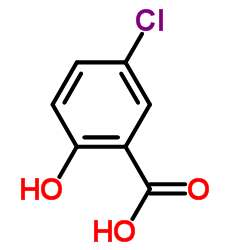
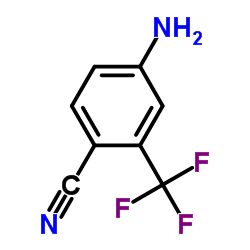
![5-chloro-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxybenzamide Structure](https://image.chemsrc.com/caspic/476/634185-61-8.png)
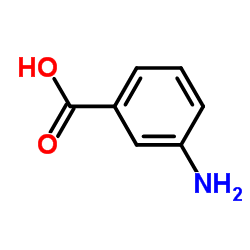
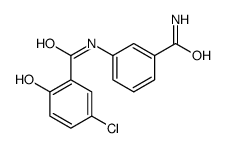


![N-[4-bromo-2-(trifluoromethyl)phenyl]-5-chloro-2-hydroxybenzamide Structure](https://image.chemsrc.com/caspic/390/634185-63-0.png)
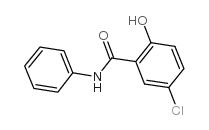

![5-chloro-N-[4-fluoro-2-(trifluoromethyl)phenyl]-2-hydroxybenzamide Structure](https://image.chemsrc.com/caspic/276/634185-66-3.png)

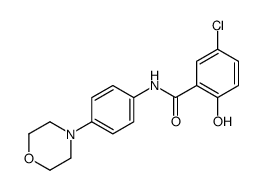

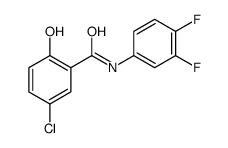

![5-chloro-2-hydroxy-N-[4-(trifluoromethoxy)phenyl]benzamide Structure](https://image.chemsrc.com/caspic/420/634186-00-8.png)