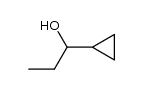
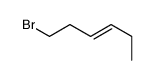
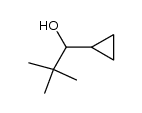
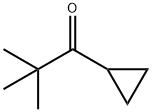
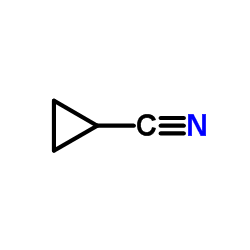
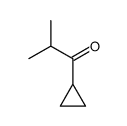
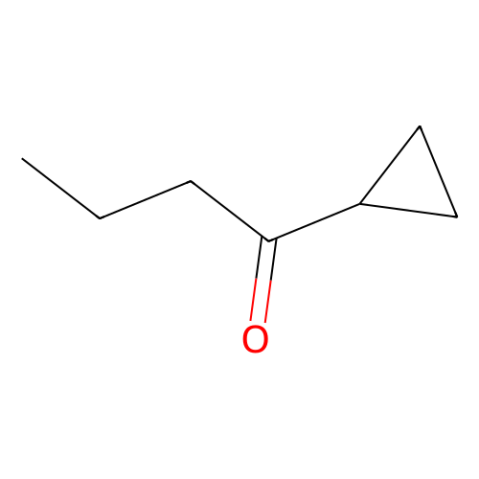
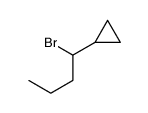
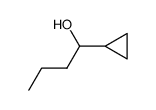
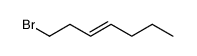
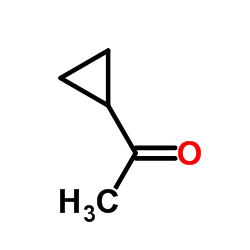
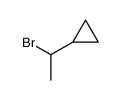
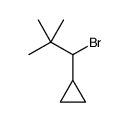
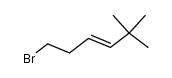
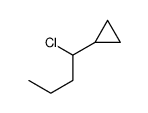
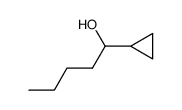
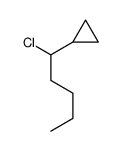
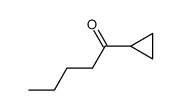
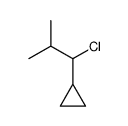
|
~% |
|
~80% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~83% |
|
~% |
|
~89% |
|
~% |
|
~96% |
|
~% |
|
~% |