|
~92% |
|
~% |
|
~% |
|
~91% |
|
~90% |
|
~% |
|
~96% |
|
~97% |
|
~% |
![Pyrido[1,2-a]benzimidazole (7CI,8CI,9CI) Structure](https://image.chemsrc.com/caspic/370/245-47-6.png)

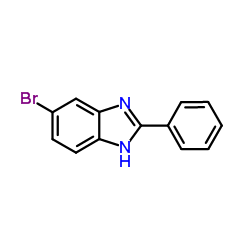
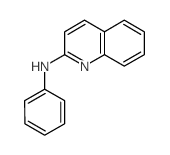
![BENZO[4,5]IMIDAZO[1,2-A]QUINOLINE Structure](https://image.chemsrc.com/caspic/057/205-54-9.png)
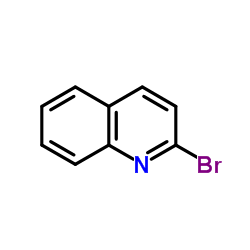
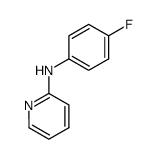
![Pyrido[1,2-a]benzimidazole, 8-fluoro- (9CI) Structure](https://image.chemsrc.com/caspic/324/136343-75-4.png)
![2-Phenyl-1H-benzo[d]imidazole Structure](https://image.chemsrc.com/caspic/350/716-79-0.png)
