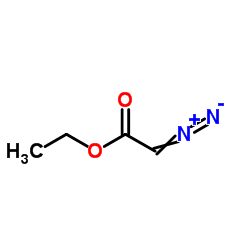
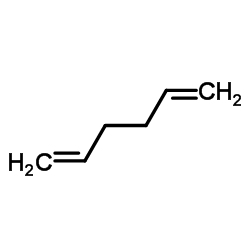
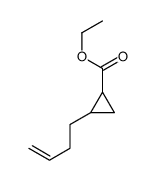
|
~54% |
|
~95% |
|
~68% |
|
~86% |
|
~82% |
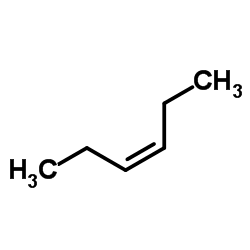
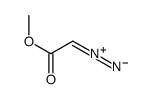

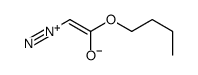
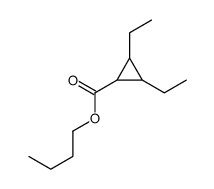
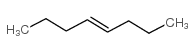
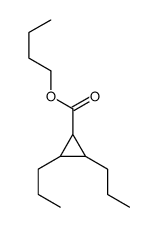

![butyl bicyclo[4.1.0]heptane-7-carboxylate Structure](https://image.chemsrc.com/caspic/385/61452-50-4.png)