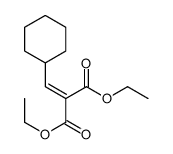
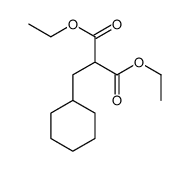
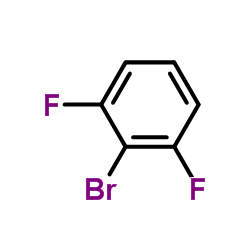
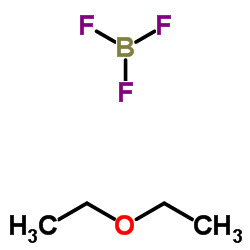
|
~96% |
|
~99% |
|
~97% |
|
~90% |
|
~60% |
![diethyl 2-[(4-fluorophenyl)methylidene]propanedioate Structure](https://image.chemsrc.com/caspic/252/790-53-4.png)
![diethyl 2-[(4-fluorophenyl)methyl]propanedioate Structure](https://image.chemsrc.com/caspic/234/59223-74-4.png)


![diethyl 2-[[4-(trifluoromethyl)phenyl]methyl]propanedioate Structure](https://image.chemsrc.com/caspic/490/29805-55-8.png)